Researchers at the Ruth Müller Lab in Terni, Italy have just taken a big step in testing the viability of genetically modified mosquitoes that could actually eradicate malaria. The lab would introduce a version of the flying insect that carries “gene drive” DNA segments, which code for sterility, to regular, non-genetically modified populations. The goal is to have the genetically modified mosquitoes mate with those who have not been genetically modified, in order to pass on their novel DNA mutations and subsequently produce sterile mosquito offspring.
The decision to introduce the genetically altered mosquitoes to unmodified populations in the lab is being highlighted as a seminal moment because it marks, according to the lab’s lead, Ruth Müller, “[the] first gene-drive release into a natural population.” Müller, who’s featured in the NPR interview above, says that she and her team don’t want to eradicate mosquitoes, but do want to control their populations in order to wipe out malaria.
The gene drives Müller is referring to are at the heart of this experiment, and are possible thanks to the relatively new disruptive genetic engineering tool known as CRISPR. CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, is actually composed of a bacterium’s defense system against bacteriophages, which are viruses that attack bacteria. Here is a quick rundown of how CRISPR works according to a Nerdist piece on designer babies:
“[CRISPR] basically operates by taking the genes of invading bacteriophages, duplicating those genes, inserting them into the [native organism’s] genome as ‘spacers’ between repeating genes (hence ‘Palindromic Repeats’) and then telling RNA (or ‘messenger RNA’ because it does the bidding of DNA in the cell) to find any DNA that matches the recorded spacers, and destroy it. In the case of purposefully engineering a genome, instead of deleting the genes of invading bacteriophages, a specific unwanted gene is made ‘the enemy’ and destroyed.”
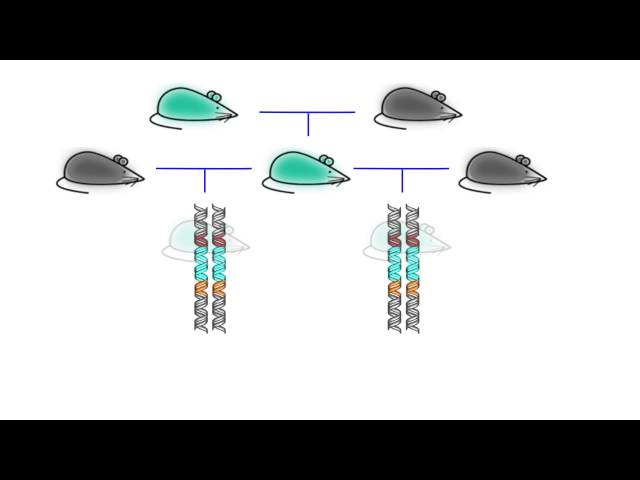
In the case of these genetically modified mosquitoes, CRISPR has been used to insert “gene drives,” which are genes that are essentially guaranteed to be passed on from one generation to another thanks to their “selfish” nature. Gene drives, in other words, have an extremely high likelihood of being passed on from one generation to the next thanks to the fact that they knock out their genetic counterpart available from the opposite parent’s genome.
In this case, Müller and her team have inserted CRISPR-based gene drives focused on producing offspring with genomes coded for infertility. This means that a genetically modified mosquito carrying the genes that cause infertility will mate with a normal mosquito, and produce DNA that has a more or less 100 percent chance of carrying the genes for infertility rather than a 50 percent chance of carrying said genes—again, because the genes allowing for fertility passed on from the unmodified parent have been knocked out by the CRISPR genes carried in the modified parent.
But while the science here is astounding and deeply complex, the ramifications of unleashing a genetically modified population of mosquitoes out in the wild are totally unknown, and could be disastrous. Although Müller doesn’t aim to totally annihilate mosquitoes from say, various African countries, where malaria is most devastating, it could very well do that.
Experts interviewed by NPR, for example, noted that gene drives are “a high-risk technology” and that they may have “unintended consequences.” Critics are ultimately saying that if native mosquito populations are inadvertently wiped out, it’s impossible to say how their nearby ecosystems will be effected. Which means these genetically altered mosquitoes could end up doing more harm than good.
But Müller and her team, which are being funded by the Bill & Melinda Gates Foundation (a foundation that also funds NPR, hence its exclusive access to the story), are obviously betting on the good of genetically modified mosquitoes outweighing the bad. Kevin Esvelt, a pioneer in this field at the Massachusetts Institute of Technology, spoke with NPR, and summed up the presumed sentiment of Müller and her team when he said that “If my kids lived in Africa, I’d say, ‘Go for [spreading the genetically modified mosquitoes] as quickly as possible.'”
What do you think of using CRISPR-based gene drives for controlling mosquito populations? Do you think this is a move in the right direction, or do the unforeseen consequences outweigh the potential benefits? Let us know in the comments!
Feature image: NPR